2.2. Future information technologies
2.2.3. Molecular electronics
Transition metal ions (TMIs) form the core units of vital catalytic process in proteins as well as in synthetic compounds. TMIs frequently exhibit high spin (HS) paramagnetic electronic ground states, as crucial catalytic intermediates. Their properties constitute not only fascinating objects of basic (bio) inorganic chemistry but render TMI complexes prime candidates for blue prints of future energy production devices. The interest in this important class of compounds even increased when the potential of HS TMI based single molecule magnets as potential nano scale data processing units was discovered. Both catalytic as well as magnetic properties result from a subtle interplay between the ion’s oxidation and spin states, their coordination geometry, interactions with surrounding ligands as well as spin-spin couplings. Although outstanding progress has been made in the synthesis of TMI complexes and their quantum chemical description, further spectroscopic knowledge is mandatory to unravel their structure-dynamic-function relationships. The vision of these efforts is the production of tailor-made systems featuring systematically tuneable properties.
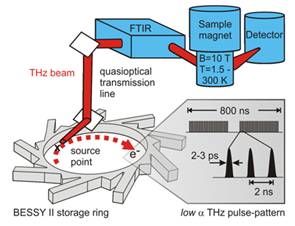
Figure 13. Quasi-optical FD-FT THz-EPR set-up consisting of radiation extraction optics in the storage ring, the quasi-optical THz transmission line, the FTIR spectrometer, the superconducting magnet with variable temperature insert (Oxford Spectromag 4000, B0 = ±11 T, T = 1.5 – 300 K), and the detector. The THz pulse pattern in low a is depicted in the gray box. The depicted excitation scheme provides sufficient power for continuous wave THz-EPR excitation, but does not allow for spin flips with a single pulse, a requirement for advanced EPR excitation. (Courtesy A. Schnegg, K. Holldack, HZB).
The ideal technique for studying paramagnetic systems is electron paramagnetic resonance (EPR) spectroscopy. However, standard EPR techniques frequently fail to detect HS TMI’s in cases where EPR transition energies exceed the quantum energy of the EPR spectrometer (typically < 4 cm-1). Very recently we demonstrated that Frequency Domain Fourier Transform (FD-FT) THz-EPR (Figure 13) based on coherent synchrotron radiation (CSR) [1] provides a unique tool to lift these restrictions. Our novel approach allows for EPR excitations over a broad energy (7 cm-1 to 35 cm-1) and magnetic field (-11 T to 11 T) range in a single spectrometer and with a single source. FD-FT THz-EPR was set-up in the frame of the BMBF funded network project EPR-Solar and is now further developed with funds from the DFG priority program 1601. It is part of the Berlin Joint EPR Lab, a common research infrastructure of Freie Universität Berlin and HZB. FD-FT THz-EPR has been successfully applied to high spin TMIs in single molecule magnets [2-5] catalytic mononuclear integer HS TMIs [5] and proteins [6]. A further drastic increase in the capabilities of CSR based THz-EPR could be achieved by the employment of ultra-short THz pulses of high repetition rate from BESSY-VSR . Following the path of state-of-the-art FT-NMR spectroscopy the availability of ultra-broad band THz-EPR experiments with sub ns time resolution would pave the way for completely new EPR experiments and access paramagnetic states relevant for (photo) catalysis and data processing. Such experiments are currently out of reach due to the fixed pulse duration (see Figure 13) and the limited THz pulse power. High power CSR THz pulses with variable duration would allow for coherent electron spin manipulation and control, laser/x-ray-pump EPR-probe experiments on function determining short lived (photo) catalytic states as well as in operando studies on HS TMIs in systems relevant for energy conversion and storage.
References for section 2.2.3.
[1] A. Schnegg et al., Phys. Chem. Chem. Phys. 11, 6820 (2009).
[2] K. S. Pedersen et al., Chem. Commun. 47, 6918 (2011).
[3] J. Dreiser et al., Chem. Eur. J. 17, 7492 (2011).
[4] J. Dreiser et al., Chem. Eur. J. 19, 3693 (2013).
[5] A. P. Forshaw et al., Inorg Chem 52, 144 (2012).
[6] J. Nehrkorn et al., submitted as invited article to Mol. Phys. (2013).