2.3. Basic energy science
2.3.4. Solar fuels
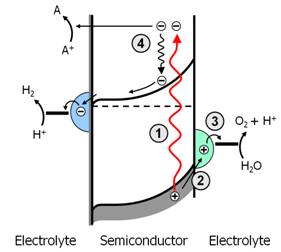
The direct conversion of abundant resources such as water and CO2 into hydrogen or hydrocarbons using sunlight provides an attractive solution for the intermittent nature of solar energy. These solar fuels have up to 100 times higher energy and power densities than the best batteries, and offer a viable solution for long-term energy storage on the required terrawatt-hour scale. Photoelectrochemistry (Figure 27) is a promising, cost-effective route towards solar fuels that combines photovoltaic and electrocatalytic functionalities within a single material or composite. The light absorbing component is a semiconductor that needs to be stable in water under strongly oxidizing and/or reducing conditions. The semiconductor is electronically coupled to an electro-catalyst, which provides the right environment for the desired chemical reactions to occur at high rates and low overpotentials.
Figure 27. Schematic representation of the photoelectrochemical processes taking places taking place during light-induced water splitting. (Courtesy R. van de Krol, HZB, and M. Bär, HZB).
Transition metal oxides are promising light absorbers that combine reasonable semiconducting properties with excellent chemical stability and low cost. Most efforts so far have focused on simple binary oxides, but efforts are currently shifting towards multinary (complex) oxides. The bulk and surface electronic structures of most of these oxides are highly complex and still poorly understood. To get a better understanding of the performance-limiting loss mechanisms it is essential to explore the charge carrier dynamics of these materials. Processes occurring on ms – ns time scales can already be accessed by standard optical and electrical spectroscopies available in many laboratories, and with the facilities available at BESSY II.
However, processes that occur on the ns – fs time scales are much harder to probe. This is especially true for transition metal oxides, whose low carrier mobilities and broad, non-distinct optical features preclude standard THz and fs transient absorption spectroscopy. BESSY-VSR would allow us to access this exciting regime, and probe the generation, recombination and charge-transfer dynamics of solar fuel materials in unprecedented detail. Some specific challenges that BESSY-VSR would be able to address are the following:
- The carrier lifetimes in many important transition metal oxides are significantly shorter than 10‑9 s. For example, the carrier lifetimes in p-type Cu2O and n-type a-Fe2O3, two of the best-known photoelectrodes for light-induced water splitting, are 147 ps and < 8 ps, respectively. Despite extensive studies, it is not yet clear whether these extremely short lifetimes are an intrinsic characteristic of the material or whether these are due to the presence of defects. A clear answer to this question is the first step towards a possible solution strategy.
- The time scales and energy losses associated with charge transfer across the semiconductor-catalyst interface are still unexplored territory. A recent perspective in Nature Chemistry on the role of a cobalt phosphate-catalyzed a-Fe2O3 surface (“Catalyst or Spectator”, Nature Chem. 4 (2012) 965) illustrates how little we know about this interface. Insight into the charge transfer dynamics will help us to understand why certain catalysts work well with some semiconductors, but not with others.
- The water oxidation reaction is often found to be the bottleneck in solar fuel devices. This is a complicated four-electron proton-coupled electron transfer process that, despite many studies, is still poorly understood. Most efforts focus on the slowest step in this process, which occurs on the ms timescale. By looking instead at much faster processes, which presumably involve momentary occupation of a hybrid orbital formed by the catalyst and chemisorbed water, additional pieces of this important four-electron transfer puzzle might be obtained.
- Hot-electron injection is a mechanism that would allow next-generation photovoltaic and solar fuel devices to break traditional theoretical performance limits. For this process to work, charge transfer to the catalyst or to an adsorbed water species should be faster than thermalization of the carriers. For a metal oxide like a-Fe2O3, thermalization of conduction band electrons occurs within 0.3 ps. This is within the temporal range offered by BESSY-VSR.