EMIL
PINK: X-Ray Emission Spectroscopy
Motivation:
From natural systems to manmade homogenous and heterogeneous catalysts, transition metals are often employed to effect challenging chemical transformations. These include the formation of oxygen-oxygen bonds, the breaking of N-N bonds and the production of methanol from methane or CO2. In all these reactions a transition metal active site is responsible for the chemical conversion. Ultimately, one would like to understand the detailed changes which occur at the active sites over the course of a reaction. Understanding the changes in the transition metal electronic structure and ligation environment is essential for any detailed mechanistic understanding, which forms the basis for rational catalysis design.
Method:
To this end, X-ray emission spectroscopy (XES) is a powerful technique. XES is an element selective technique which provides local structural information near the selected atomic species[1]. XES measurements and analysis of x-ray emission lines can reveal the ligand identity, the ligand ionization potential, and the metal-ligand distance. XES can also help to identify oxidation states of the active components[2,3]. In contrast to photoelectron spectroscopy (PES), XES is a photon-in-photon-out technique, thus the probing depth is roughly two orders of magnitude larger than in PES.
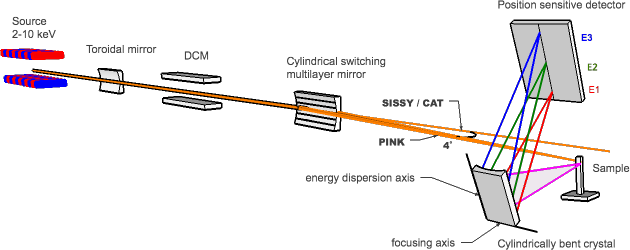
The PINK branch at the EMIL beamline will be designed to operate in the hard x-rays regime with energies ranging from 2 to 10 keV. This unique energy range will provide access to non-resonant XES studies of transition metals ranging from Ti to Cu (Kα, Kβ lines) and Zr to Ag (Lα, Lβ lines), as well as light elements P, S, Cl, K, Ca (Kα, Kβ lines). Capabilities for resonant XES are currently under consideration.
The PINK beamline aims to be robust and simple to operate. Since XES is a "photon-hungry" technique a high throughput multilayer monochromator will focus the beam in a 20μmx500 μm (VxH) spot and provide the high photon flux (1014 ph/s @ 6 keV) at the sample allowing measurements of very dilute substances. Fluorescent X-rays will be analyzed by a wavelength-dispersive von Hamos crystal spectrometer. Taking advantage of a wavelength-dispersive analyzer to record the entire spectrum simultaneously, we will be able to provide very attractive time-resolved (0.1 -10 s) XES measurements[4]. The experimental set-up will be highly automated, both in terms of sample handling hardware and data collection.
The PINK branch will be designed, constructed and operated by the Max-Planck-Institute für Chemische Energiekonversion in close collaboration with Helmholtz-Zentrum Berlin für Materialien und Energie GmbH.
Where to learn more:
[1] U. Bergmann and P. Glatzel, "X-ray emission spectroscopy", Photosynth. Res., 255 (2009)
[2] M. A. Beckwith, M. Roemelt, M.-N. Collomb, C. DuBoc, T.-C. Weng U. Bergmann, P. Glatzel, F. Nesse and S. DeBeer, "Manganese Kb x-ray emission spectroscopy as a probe of metal-ligand interactions", Inorg. Chem, 50, 8397, (2011)
[3] C. J. Pollock, K. Grubel, P. H. Holland, S. DeBeer, "Experimentally quantifying small-molecule bond activation using valence-to-core x-ray emission spectroscopy", J. of the Am. Chem. Soc., 135, 32, 11803 (2013)
[4] J. Szlachetko, M. Nachtegaal, E. de Boni, M. Willimann, O. Safonova, J. Sa, G. Smolentsev, M. Szlachetko, J. A. van Bokhoven, J.-Cl. Dousse, J. Hoszowska, Y. Kayser, P. Jagodzinski, A. Bergamaschi, B. Schmitt, C. David, and A. Lücke, "A von Hamos x-ray spectrometer based on a segmented-type diffraction crystal for single-shot x-ray emission spectroscopy and time-resolved resonant inelastic x-ray scattering studies", Rev. Sci. Inst., 83, 103105 (2012)