Göttingen scientists use BESSY II to decode basic mechanism underlying biochemical reactions
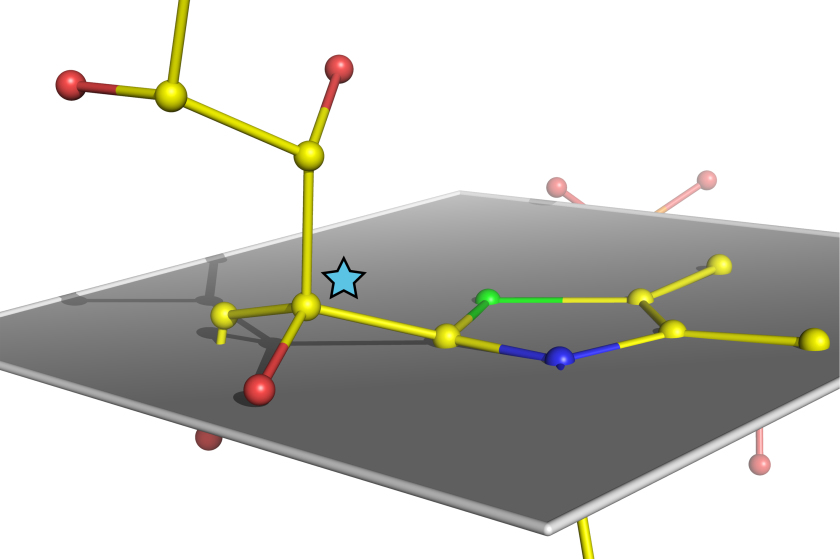
Structure of the sugar molecule bound by the enzyme transketolase immediately prior to its being split
Enzymes are life’s molecular catalysts and figure prominently in cellular metabolism. It has been speculated that in the course of a biochemical reaction enzymes physically bend their substrates to split them. Now for the first time ever, scientists at the Göttingen Center for Molecular Biosciences (GZMB) have successfully used BESSY II's MX beamline to unequivocally confirm this hypothesis. The results from this study have been published in the renowned scientific journal Nature Chemistry.
The Göttingen team around Prof. Dr. Kai Tittmann and Prof. Dr. Ralf Ficner started out by growing high order protein crystals of the human enzyme transketolase, which plays a central role in human metabolism during sugar processing. Natural sugar substrates were added to the protein crystals. Analysis of the enzyme’s crystalline structure was subsequently performed at electron storage ring BESSY II's MX beamline and in French Grenoble. The scientists were able to determine the structure of the sugar molecule bound by the enzyme immediately prior to its being split in half at an ultrahigh spatial resolution of 0.1 nanometers. “The snapshot we got of an enzyme at work, which really is unprecedented in terms of resolution, unequivocally reveals how the sugar substrate is being bent by the enzyme, similar to a vise clamping a work piece,” Prof. Tittmann explains.
In many cases, enzymes are drug targets. Which is why these new insights are important for the development of customized, highly specific active substances like those used in cancer therapy. “Even the human transketolase used in this study plays a key role in cancer cell metabolism,” says Prof. Tittmann.
Source: Göttingen University
https://www.helmholtz-berlin.de/pubbin/news_seite?nid=13800;sprache=en
- Copy link
-
Green fabrication of hybrid materials as highly sensitive X-ray detectors
New bismuth-based organic-inorganic hybrid materials show exceptional sensitivity and long-term stability as X-ray detectors, significantly more sensitive than commercial X-ray detectors. In addition, these materials can be produced without solvents by ball milling, a mechanochemical synthesis process that is environmentally friendly and scalable. More sensitive detectors would allow for a reduction in the radiation exposure during X-ray examinations.
-
Electrical energy storage: BAM, HZB, and HU Berlin plan joint Berlin Battery Lab
The Federal Institute for Materials Research and Testing (BAM), the Helmholtz-Zentrum Berlin (HZB), and Humboldt University of Berlin (HU Berlin) have signed a memorandum of understanding (MoU) to establish the Berlin Battery Lab. The lab will pool the expertise of the three institutions to advance the development of sustainable battery technologies. The joint research infrastructure will also be open to industry for pioneering projects in this field.
-
BESSY II: Insight into ultrafast spin processes with femtoslicing
An international team has succeeded at BESSY II for the first time to elucidate how ultrafast spin-polarised current pulses can be characterised by measuring the ultrafast demagnetisation in a magnetic layer system within the first hundreds of femtoseconds. The findings are useful for the development of spintronic devices that enable faster and more energy-efficient information processing and storage. The collaboration involved teams from the University of Strasbourg, HZB, Uppsala University and several other universities.
