Corona research: Consortium of Berlin research and industry seeks active ingredients
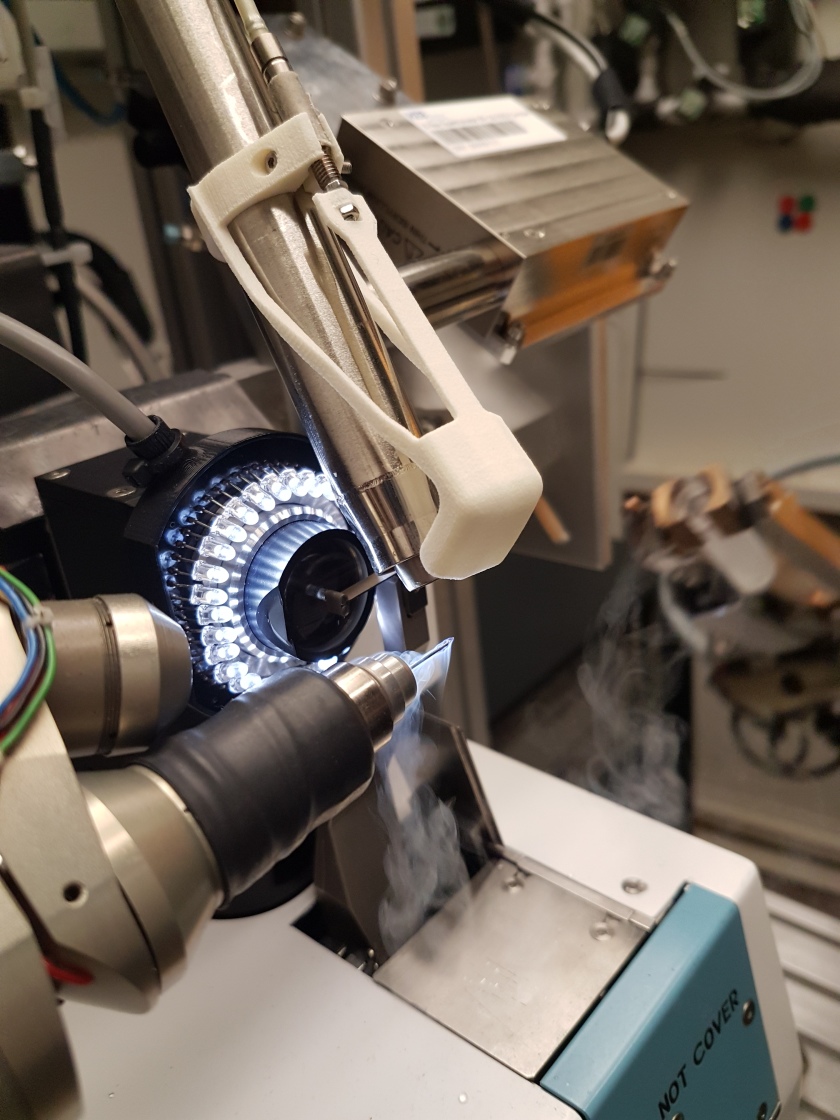
Protein crystals are analysed in the MX laboratory at BESSY II with hard X-rays. © C. Feiler/HZB
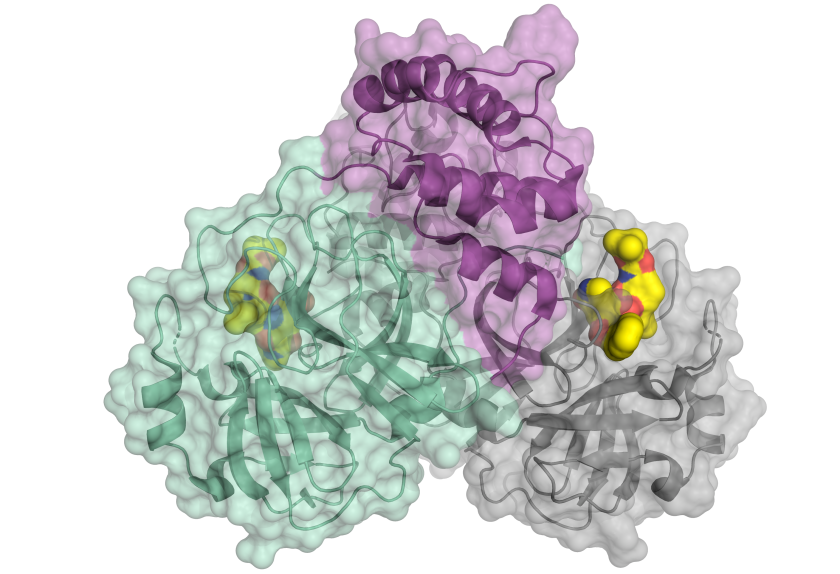
At BESSY II, Prof. Rolf Hilgenfeld (Uni Lübeck) was able to analyse an important protein of the SARS-CoV2 virus, the viral main protease which enables the virus to multiply. © H.Tabermann/HZB
The Berlin biotech company Molox GmbH and a team at the Helmholtz-Zentrum Berlin (HZB) have initiated a consortium of regional research groups and BASF. Together, they want to identify a starting point for the development of a potential active substance against the new coronavirus. Targets of potential inhibitors will be SARS-CoV2 proteins that promote the spread or infectivity of the viruses. Scientists from Freie Universität Berlin are also involved in the research work.
"Berlin combines important large-scale infrastructure with an excellent network of academic and industrial structural biologists and biochemists. The distances here are short, but resources and expertise must be strategically coordinated to be successful," says Dr. Holger von Moeller, the owner of the biotech company Molox.
Access to synchrotron radiation is essential for the success of the project. This particularly intense radiation is provided by the Berlin Electron Storage Ring for Synchrotron Radiation (BESSY II), which is operated by the HZB.
Several research groups at Freie Universität Berlin led by Prof. Markus Wahl, Prof. Christian Freund, Dr. Ursula Neu, and Prof. Sutapa Chakrabarti are working with Molox to produce the proteins and then crystallize them.
"The HZB is making all existing infrastructures available to the joint project," explains Dr. Manfred Weiss, head of the Research Group Macromolecular Crystallography (MX) at HZB.
BASF is the first project partner from the chemical industry to provide funds to start the investigations. Protein crystals will be saturated with potential inhibitors and subsequently analysed on the MX beamlines of BESSY II. In this way it can be discovered which compounds are particularly good at inhibiting the function of the protein - these should then be the starting points for the development of active substances.
The consortium is currently negotiating with other partners in order to acquire them and their substance libraries. "We are looking forward to this joint project and hope that we will be able to identify new potential active substances against SARS-CoV-2 very quickly", says Dr. Christian Feiler, project leader at HZB.
red.
https://www.helmholtz-berlin.de/pubbin/news_seite?nid=21283;sprache=en
- Copy link
-
Protein crystallography at BESSY II: faster, better and more and more automatic
Many diseases are linked to malfunctions of proteins in the organism. The three-dimensional architecture of these molecules is often highly complex, but it can provide valuable insights into biological processes and the development of drugs. X-ray diffraction at the MX beamlines of BESSY II can be used to decipher the 3D structure of proteins. To date, more than 5000 structures have been solved at the three MX beamlines. Here, we present a review and an outlook with Manfred Weiss, head of the research group for macromolecular crystallography.
-
5000th protein structure at BESSY II: Starting point for a COVID drug
Many proteins have a complex architecture that enables biological functions. Molecules can bind to specific sites on a protein and alter its function. A team at HZB has now investigated the Nsp1 protein, which plays a role in infection with the SARS-CoV-2 virus. They analysed protein crystals, previously mixed with molecules from a fragment library, and discovered a total of 21 candidates as starting points for drug development. At the same time, they also decoded the 5000th structure at BESSY II.
-
What Zinc concentration in teeth reveals
Teeth are composites of mineral and protein, with a bulk of bony dentin that is highly porous. This structure is allows teeth to be both strong and sensitive. Besides calcium and phosphate, teeth contain trace elements such as zinc. Using complementary microscopy imaging techniques, a team from Charité Berlin, TU Berlin and HZB has quantified the distribution of natural zinc along and across teeth in 3 dimensions. The team found that, as porosity in dentine increases towards the pulp, zinc concentration increases 5~10 fold. These results help to understand the influence of widely-used zinc-containing biomaterials (e.g. filling) and could inspire improvements in dental medicine.
