User research at BESSY II: Graphite electrodes for rechargeable batteries investigated
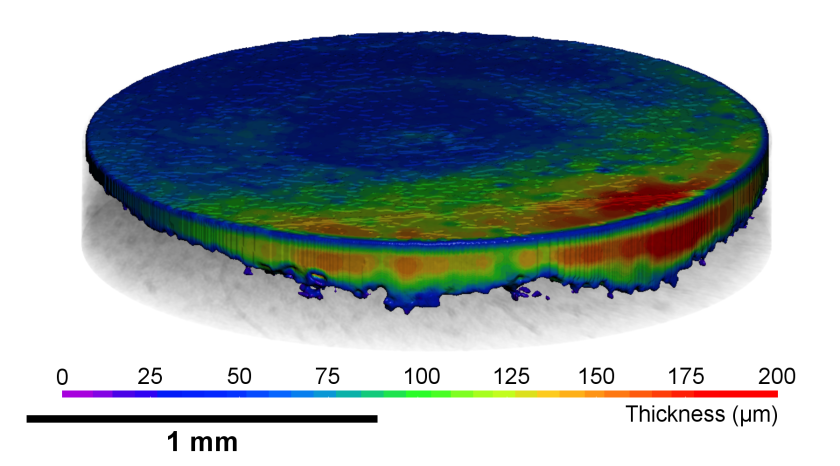
The tomogram during the charging process shows the spatially resolved changes in the graphite electrode thickness of a rechargeable aluminium ion battery in a discharged and charged state. © HZB
Rechargeable graphite dual ion batteries are inexpensive and powerful. A team of the Technical University of Berlin has investigated at the EDDI Beamline of BESSY II how the morphology of the graphite electrodes changes reversibly during cycling (operando). The 3D X-ray tomography images combined with simultaneous diffraction now allow a precise evaluation of the processes, especially of changes in the volume of the electrodes. This can help to further optimise graphite electrodes.
Published in Advanced Functional Materials (2020); Simultaneous X‐Ray Diffraction and Tomography Operando Investigation of Aluminum/Graphite Batteries; Giuseppe Antonio Elia, Giorgia Greco, Paul Hans Kamm, Francisco García‐Moreno, Simone Raoux, Robert Hahn
DOI: 10.1002/adfm.202003913
Abstract: Rechargeable graphite dual‐ion batteries are extremely appealing for grid‐level stationary storage of electricity, thanks to the low‐cost and high‐performance metrics, such as high‐power density, energy efficiency, long cycling life, and good energy density. An in‐depth understanding of the anion intercalation mechanism in graphite is fundamental for the design of highly efficient systems. In this work, a comparison is presented between pyrolytic (PG) and natural (NG) graphite as positive electrode materials in rechargeable aluminum batteries, employing an ionic liquid electrolyte. The two systems are characterized by operando synchrotron energy‐dispersive X‐ray diffraction and time‐resolved computed tomography simultaneously, establishing a powerful characterization methodology, which can also be applied more in general to carbon‐based energy‐related materials. A more in‐depth insight into the AlCl4−/graphite intercalation mechanism is obtained, evidencing a mixed‐staged region in the initial phase and a two‐staged region in the second phase. Moreover, strain analysis suggests a correlation between the irreversibility of the PG electrode and the increase of the inhomogenous strain. Finally, the imaging analysis reveals the influence of graphite morphology in the electrode volume expansion upon cycling.
red.
https://www.helmholtz-berlin.de/pubbin/news_seite?nid=22334;sprache=en
- Copy link
-
Fascinating archaeological find becomes a source of knowledge
The Bavarian State Office for the Preservation of Historical Monuments (BLfD) has sent a rare artefact from the Middle Bronze Age to Berlin for examination using cutting-edge, non-destructive methods. It is a 3,400-year-old bronze sword, unearthed during archaeological excavations in Nördlingen, Swabia, in 2023. Experts have been able to determine how the hilt and blade are connected, as well as how the rare and well-preserved decorations on the pommel were made. This has provided valuable insight into the craft techniques employed in southern Germany during the Bronze Age. The BLfD used 3D computed tomography and X-ray diffraction to analyse internal stresses at the Helmholtz-Zentrum Berlin (HZB), as well as X-ray fluorescence spectroscopy at a BESSY II beamline supervised by the Bundesanstalt für Materialforschung und -prüfung (BAM).
-
Element cobalt exhibits surprising properties
The element cobalt is considered a typical ferromagnet with no further secrets. However, an international team led by HZB researcher Dr. Jaime Sánchez-Barriga has now uncovered complex topological features in its electronic structure. Spin-resolved measurements of the band structure (spin-ARPES) at BESSY II revealed entangled energy bands that cross each other along extended paths in specific crystallographic directions, even at room temperature. As a result, cobalt can be considered as a highly tunable and unexpectedly rich topological platform, opening new perspectives for exploiting magnetic topological states in future information technologies.
-
MXene for energy storage: More versatile than expected
MXene materials are promising candidates for a new energy storage technology. However, the processes by which the charge storage takes place were not yet fully understood. A team at HZB has examined, for the first time, individual MXene flakes to explore these processes in detail. Using the in situ Scanning transmission X-ray microscope 'MYSTIIC' at BESSY II, the scientists mapped the chemical states of Titanium atoms on the MXene flake surfaces. The results revealed two distinct redox reactions, depending on the electrolyte. This lays the groundwork for understanding charge transfer processes at the nanoscale and provides a basis for future research aimed at optimising pseudocapacitive energy storage devices.
