Department Materials Chemistry for Catalysis
Research
Research Vision and Philosophy
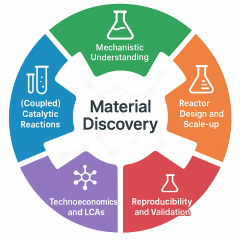
At the core of our research lies a fundamental belief: that the tools of inorganic and materials chemistry hold immense potential to engineer advanced catalysts and catalytic processes essential for a sustainable future. We are driven by the idea that through thoughtful design, we can create catalysts that not only perform exceptionally well but also meet the practical demands of real-world applications. Our goal is to establish a versatile platform of molecularly derived and solid-state catalysts, built on a foundation that integrates synthetic creativity, deep mechanistic understanding, and technological relevance. These catalysts will be designed to operate efficiently under mild, energy-efficient conditions, make use of earth-abundant and non-toxic materials, and remain stable and effective over long operational lifetimes. To achieve this, my research strategy is built on multiple interconnected pillars: catalyst discovery and rational design, integration of coupled catalytic processes, mechanistic understanding through in-situ/operando characterization, reactor and cell design with scale-up for industrial relevance, and technoeconomic and life cycle analysis to evaluate sustainability and cost-effectiveness. A critical component of this framework is a strong emphasis on reproducibility and transparency, ensuring the development of robust, standardized protocols for synthesis and catalysis. Taken together, this integrated approach ensures that catalyst development is guided not only by chemical intuition but also by data-driven insight into scalability, industrial feasibility, and long-term impact, bridging the gap between laboratory and industrial application.
1. Rational Design and Discovery of Catalytic Materials
Our laboratory focuses on the design and discovery of a broad spectrum of catalyst materials, spanning metals, intermetallics, alloys, metal oxides, metal hydroxides, metal carbides, nitrides, phosphides, sulfides, selenides, borides, and hybrid or template-assisted carbon-based materials and single-atom catalysts. We employ state-of-the-art synthetic methodologies including arc melting, high-temperature annealing, template-assisted pyrolysis, thin film deposition, galvanic replacement, ball milling, microwave-assisted synthesis, wet chemical reduction/oxidation, electrodeposition, co-precipitation, colloidal, and solvothermal methods to control synthetic properties such as composition, phase, crystallinity, defect density, oxidation state, coordination environment, morphology, particle size, and electrical conductivity.
By systematically linking these synthetic properties to electrochemical catalytic properties including the nature and number of active sites, charge and mass transport, reaction microenvironment, and durability we establish predictive design principles for high-performance electrocatalysts. Recognizing that many synthesized materials serve as precatalysts undergoing in situ reconstruction during electrochemical operation, we investigate how initial material properties dictate the emergence and evolution of the true active phase, enabling rational control over activity, selectivity, and stability.
2. (Coupled) Electrocatalytic Processes
(i). Electrochemical Water Splitting and Renewable Hydrogen Production
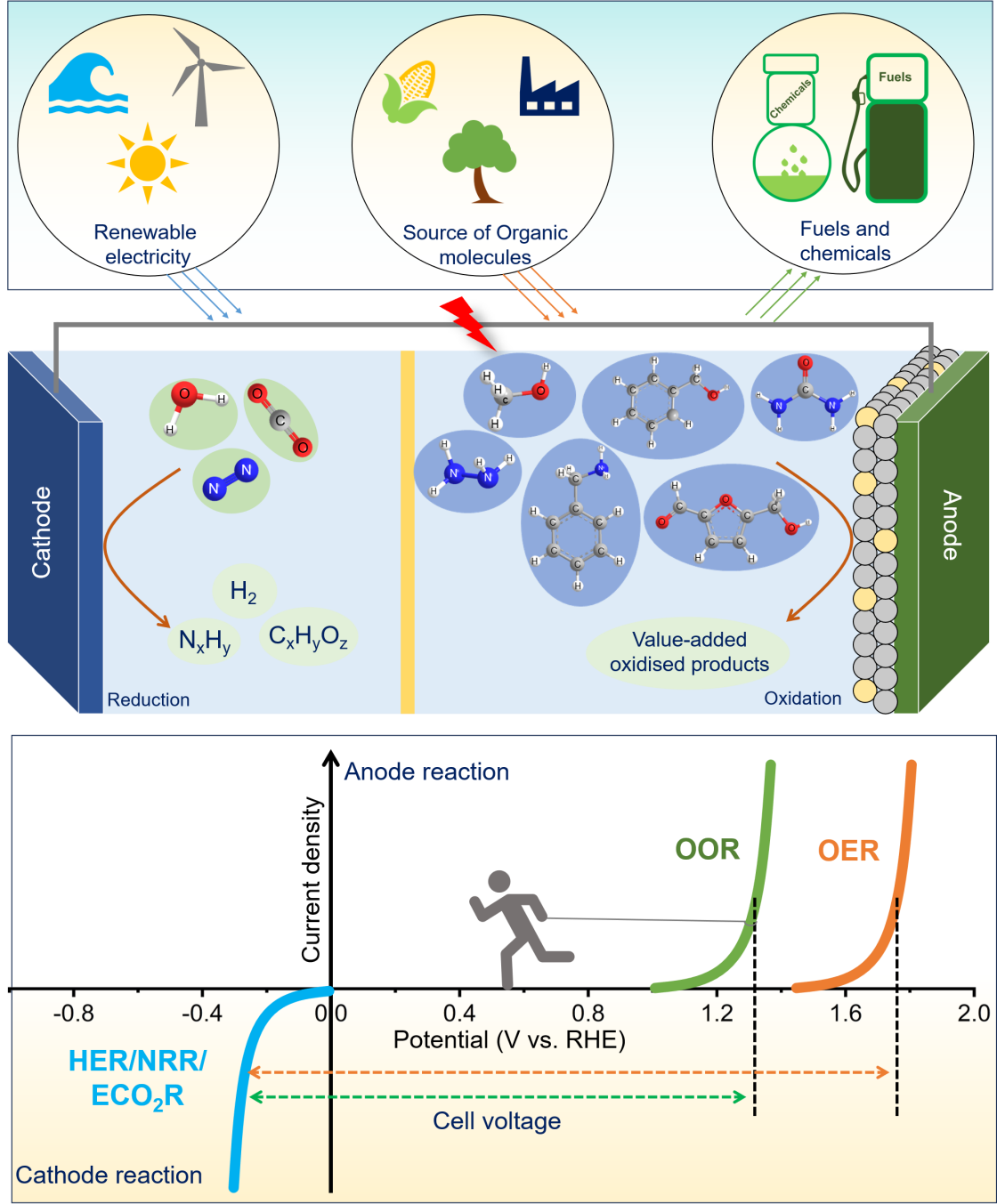
Our research aims to optimize water electrolysis across both alkaline and proton-exchange platforms, focusing on the rational tuning of active sites and local reaction environments to minimize overpotentials and enhance long-term stability. We also explore hybrid electrolyzers, designing materials and cell architectures that allow efficient integration with renewable electricity and facilitate scalable hydrogen production with low energy footprints.
(ii). Electrochemical Valorization of Biomass and Organic Waste
We develop catalytic systems for the selective electrooxidation of biomass (5-hydroxymethylfurfural, HMF), plastic-derived (ethylene glycol), and crude oil-derived feedstocks (like glycerol) into high-value chemicals, leveraging controlled reaction environments and tailored active sites. By integrating low-potential selective oxidation pathways, our work seeks to convert complex organic waste streams into energy-dense and chemically valuable products while minimizing competing reactions.
(iii). Selective Electrocatalytic Reductions: Carbon Dioxide Reduction and Hydrogen Peroxide Synthesis
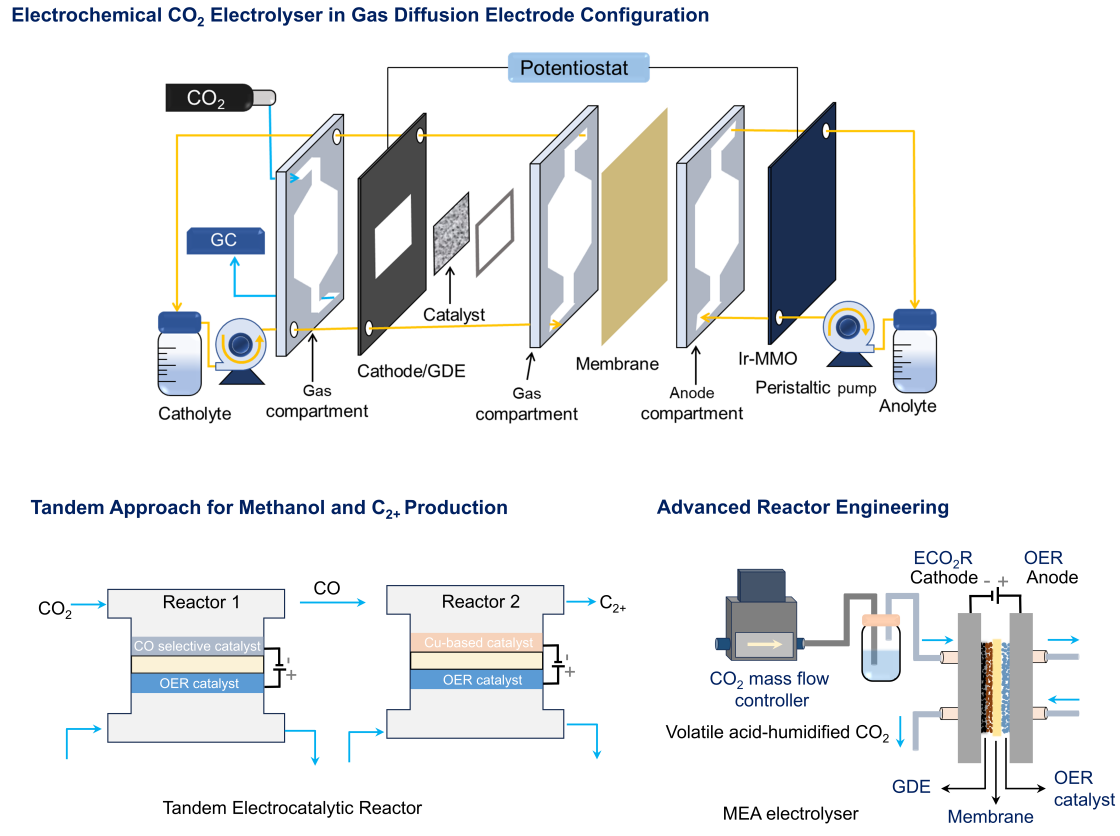
We design and engineer catalysts for highly selective CO2 electroreductions in gas diffusion electrode configuration in flow cell, targeting products such as CO (on Ag-based catalysts), formate (on Bi-based catalysts), multi-carbon chemicals like methanol and ethylene (on Cu-based catalysts).
We investigate the two-electron oxygen reduction reaction for H2O2 production, addressing key challenges such as controlling selectivity toward H2O2 over water, overcoming mass transport limitations in gas-diffusion electrodes, and designing active sites that favor the desired reaction pathway.
3. Operando Characterization and Mechanistic Elucidation
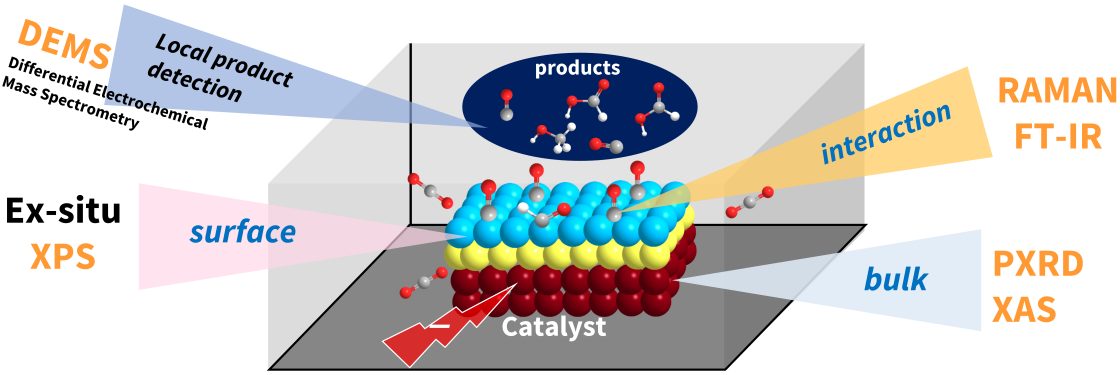
Our laboratory employs operando spectroscopic and electrochemical techniques including X-ray absorption spectroscopy (XAS), Raman, FTIR, and differential electrochemical mass spectrometry (DEMS) to monitor dynamic structural and chemical transformations of catalytic materials under reaction conditions. By correlating real-time surface and bulk evolution with electrochemical performance, we elucidate mechanistic pathways, active site dynamics, and phase transformations, providing predictive insights that guide the rational design of more robust and efficient catalysts.
4. Reactor/Cell Design and Device Scale-Up
We explore the translation of catalytic materials to functional devices, emphasizing the coupling of catalyst properties with cell architecture, mass transport optimization, and scalable reactor design. Our laboratory operates anion exchange membrane electrolyzers (5–20 cm2) for water electrolysis and CO2 electrolyzers (1–10 cm2), enabling systematic evaluation of material performance under device-relevant conditions. We also investigate tandem electrolysis configurations, integrating sequential reactions to enhance CO2 conversion efficiency and product selectivity. By combining fundamental insights from materials and electrochemistry with engineering principles, we aim to bridge the gap between laboratory-scale discovery and industrially relevant electrocatalytic systems, facilitating the practical deployment of sustainable energy and chemical conversion technologies.
5. Techno-economic and Life Cycle Analysis
Beyond material performance, our research evaluates the economic viability and sustainability of electrocatalytic processes through techno-economic modeling and life cycle assessments. By quantifying energy consumption, raw material requirements, environmental impact, and overall process efficiency, we provide a framework for sustainable technology translation, ensuring that our innovations are not only scientifically robust but also socially and environmentally responsible.
6. Reproducibility, Benchmarking, and Data-Driven Analysis
We emphasize rigorous benchmarking and reproducibility by standardizing synthetic protocols, electrochemical testing conditions, and performance metrics. Coupled with data-driven analytics and machine learning, our approach enables the identification of robust structure-performance relationships, accelerates catalyst discovery, and fosters transparency in electrocatalysis research. Operando tracking of precatalyst-to-active phase evolution ensures that dynamic structural transformations are consistently accounted for, providing reproducible and mechanistically meaningful insights.