4000th protein structure decoded at BESSY II
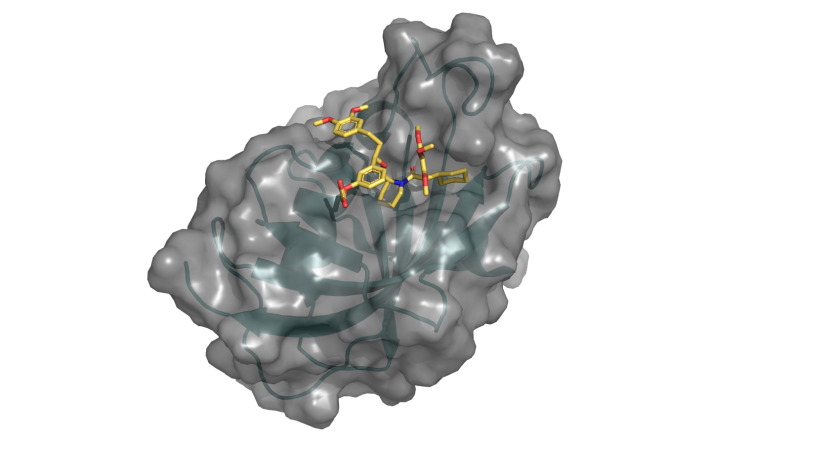
The 4000th protein structure from HZB BESSY published in PDB shows the G64S variant of FKBP51 in complex with the highly selective ligand SAFit (marked structure). © C. Meyners/TU Darmstadt/HZB
The 4000th structure is the molecule FKBP51, which is linked to stress-induced diseases such as depression, chronic pain and diabetes. The team led by Prof. Felix Hausch, TU Darmstadt, is using the knowledge of the three-dimensional structure to develop new strategies for the design of suitable drugs.
Many diseases are related to malfunctions of proteins in the organism. The three-dimensional architecture of these molecules is often extremely complex, but provides valuable clues as to how the malfunction could be remedied, for example by drugs that bind perfectly into a "pocket" on the target molecule and block the malfunction. The structure of proteins can be deciphered with X-ray analyses at the MX beamlines of BESSY II.
The 4000th structure from BESSY II has now been entered in the Protein Data Bank (www.rcsb.org/pdb), which contains all experimentally determined protein structures. The team led by Prof. Felix Hausch from TU Darmstadt had produced protein crystals from the molecule FKBP51 and examined them on the MX beamlines.
This is a protein that plays a special role in major health problems of our time. FKBP51 regulates the signal transduction of steroid hormone receptors, which can be disturbed by stress. This can trigger depression, chronic pain or diseases such as diabetes and obesity. The protein FKBP51 has shown promise as a target for drugs against these diseases. "Protein structure analysis shows us where interesting "pockets" are located in the molecule that could be drug targets," says Dr. Christian Meyners from the TU Darmstadt team.
arö
https://www.helmholtz-berlin.de/pubbin/news_seite?nid=24367;sprache=en
- Copy link
-
Alternating currents for alternative computing with magnets
A new study conducted at the University of Vienna, the Max Planck Institute for Intelligent Systems in Stuttgart, and the Helmholtz Centers in Berlin and Dresden takes an important step in the challenge to miniaturize computing devices and to make them more energy-efficient. The work published in the renowned scientific journal Science Advances opens up new possibilities for creating reprogrammable magnonic circuits by exciting spin waves by alternating currents and redirecting these waves on demand. The experiments were carried out at the Maxymus beamline at BESSY II.
-
Key role of nickel ions in the Simons process discovered
Researchers at the Federal Institute for Materials Research and Testing (BAM) and Freie Universität Berlin have discovered the exact mechanism of the Simons process for the first time. The interdisciplinary research team used the BESSY II light source at the Helmholtz Zentrum Berlin for this study.
-
IRIS beamline at BESSY II extended with nanomicroscopy
The IRIS infrared beamline at the BESSY II storage ring now offers a fourth option for characterising materials, cells and even molecules on different length scales. The team has extended the IRIS beamline with an end station for nanospectroscopy and nanoimaging that enables spatial resolutions down to below 30 nanometres. The instrument is also available to external user groups.
