Institute Electrochemical Energy Storage
Operando
Operando Small-Angle-Scattering
Operando analysis of Li2S formation with small-angle neutron scattering at BER II
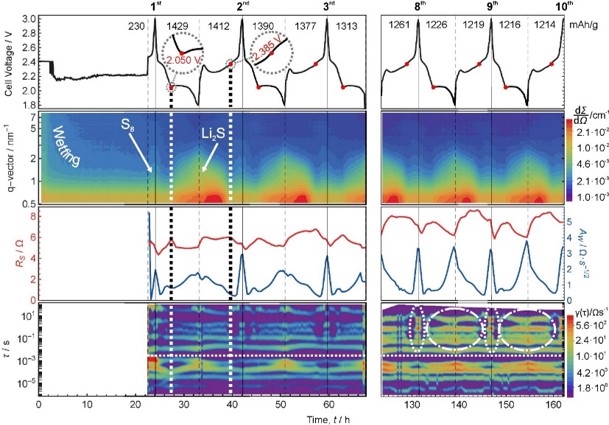
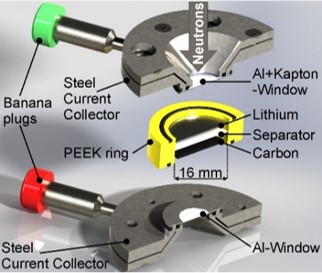
This study reports the use of operando small-angle neutron scattering to investigate processes in an operating Li/S battery. The combination with impedance spectroscopy yields valuable insights into the precipitation and dissolution of lithium
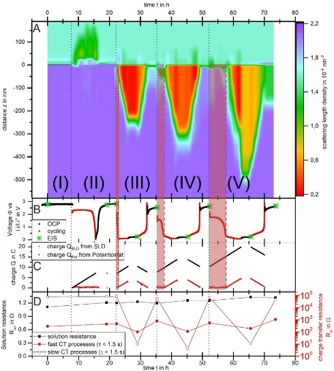
sulfide during 10 cycles of galvanostatic cycling. The use of a deuterated electrolyte increases strongly the sensitivity to detect the sulfur and Li2S precipitates at the carbon host electrode and allows us to observe the time-dependent initial wetting of the system. No correlation of the scattering signal of the micropores with either lithium sulfide or sulfur is observable during the whole course of the experiment. Hence both reaction products do not precipitate inside the microporous structure but on the outer surface of the micrometer-sized carbon fibers used in this study. The excellent scattering contrast allows a detailed analysis of the formation and dissolution process of nanoscopic Li2S structures. While lithium sulfide particles rowhomogeneously during the precipitation period, smaller Li2S particles dissolve first followed by a sudden dissolution of the larger Li2S particles.
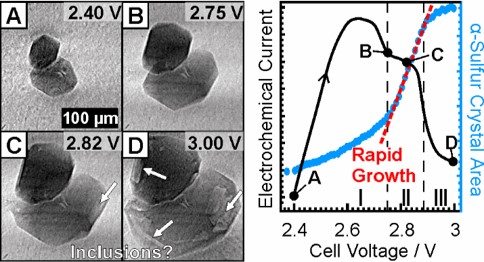
Operando analysis of S8 formation with X-Ray Imaging at BESSY II
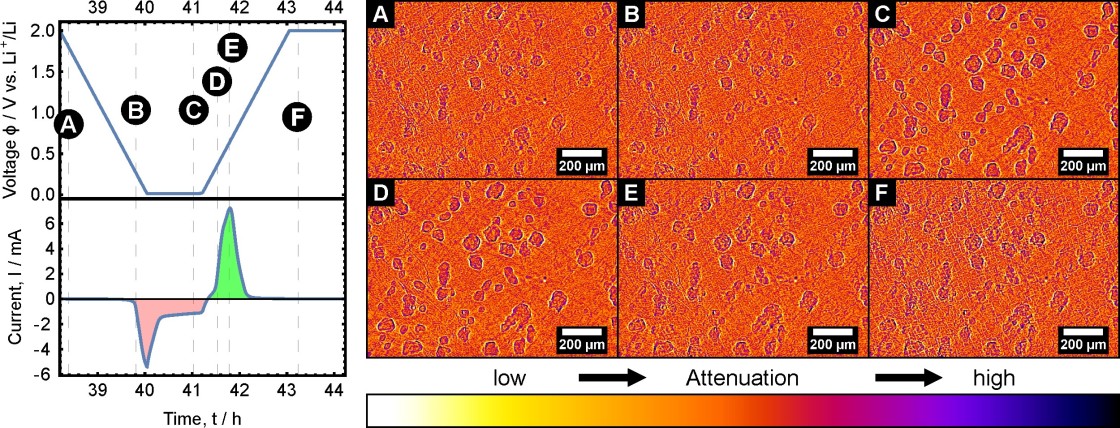
Herein, we present a detailed investigation of the electrochemically triggered formation and dissolution processes of α- and β-sulfur crystals on a monolithic carbon cathode using operando high-resolution synchrotron radiography (438 nm/pixel). The combination of visual monitoring with the electrical current response during cyclic voltammetry provides valuable insights into the sulfur formation and dissolution mechanism. Our observations show that the crystal growth process is mainly dictated by a rapid equilibrium between long-chain polysulfides on one side and solid sulfur/short-chain polysulfides on the other side, which is consistent with previous studies in this field. The high temporal and spatial resolution of synchrotron imaging enables the observation of different regimes during the sulfur formation and dissolution process. The appearance of short-chain polysulfides after the first anodic CV peak initiates a rapid dissolution process of α-sulfur crystals on the cathode. The increase in the long-chain lithium polysulfide concentration at the cathode surface during charge results in an increased crystal growth rate, which in turn produces imperfections in α- and β-sulfur crystals. There are strong indications that these defects are fluid inclusions, which may trap dissolved polysulfides and therefore reduce the electrochemical cell capacity.
Operando analysis of crystalline silicon electrode with X-Ray Imaging at BESSY II
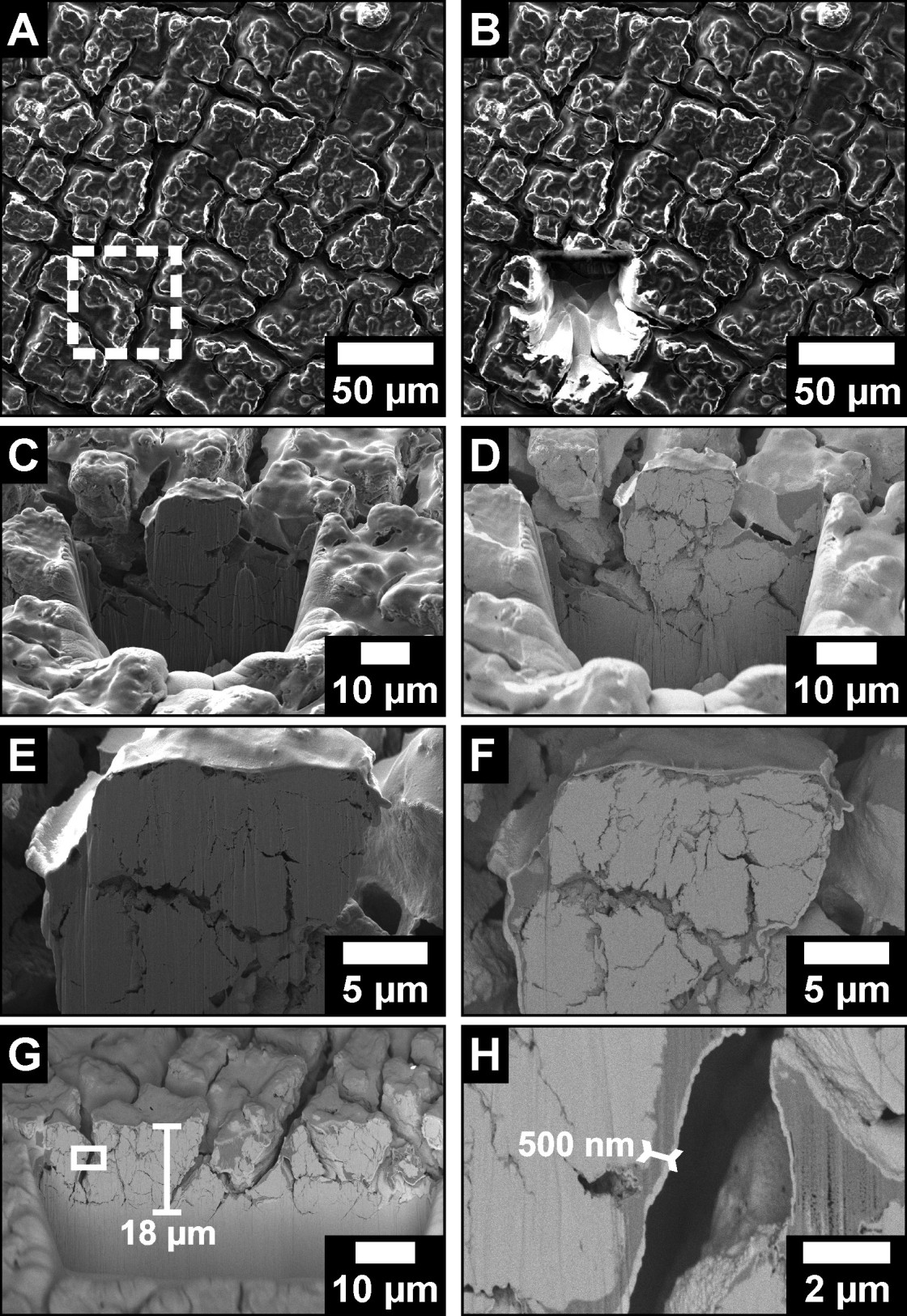
Operando phase-contrast radiography combined with impedance spectroscopy and electron microscopy is applied to study the morphological changes in a lithium-silicon cell over several cycles. The single-crystal silicon (100) surface is employed as a working electrode. A checkerboard-like cracking pattern aligned with the crystallographic axis is formed in the 4th cycle during the second step of the delithiation. With the crack position kept fixed, this crack pattern vanishes during lithiation and gets more pronounced in the subsequent cycles. This pattern forms just after the first delithiation peak that corresponds to the decomposition of the highly lithiated phase. It vanishes during lithiation in the potentiostatic step. Therefore, capacity-limited cycling, which partly maintains the highly lithiated phase, may avoid the formation of fractures. The surface area of the crack cells remains almost constant with increasing cycle numbers, and no electrochemically inactive sites were observed.
Operando Neutron Reflektometry of Si Anodes
Operando Neutron Reflectometry
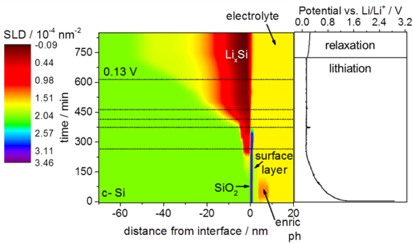
We present an operando neutron reflectometry study on the electrochemical incorporation of lithium into crystalline silicon for battery applications. Neutron reflectivity is measured from the ⟨100⟩ surface of a silicon single crystal which is used as a negative electrode in an electrochemical cell. The strong scattering contrast between Si and Li due to the negative scattering length of Li leads to a precise depth profile of Li within the Si anode as a function of time. The operando cell can be used to study the uptake and the release of Li over several cycles. Lithiation starts with the formation of a lithium enrichment zone during the first charge step. The uptake of Li can be divided into a highly lithiated zone at the surface (skin region) (x ∼ 2.5 in LixSi) and a much less lithiated zone deep into the crystal (growth region) (x ∼ 0.1 in LixSi). The total depth of penetration was less than 100 nm in all experiments. The thickness of the highly lithiated zone is the same for the first and second cycle, whereas the thickness of the less lithiated zone is larger for the second lithiation. A surface layer of lithium (x ∼ 1.1) remains in the silicon electrode after delithiation. Moreover, a solid electrolyte interface is formed and dissolved during the entire cycling. The operandoanalysis presented here demonstrates that neutron reflectivity allows the tracking of the kinetics of lithiation and delithiation of silicon with high spatial and temporal resolution.
Silicon is a promising anode material for lithium ion batteries due to its ten times higher specific capacity compared to commercially used graphite anodes. However, silicon anodes suffer from strong capacity fading and low Coulombic efficiency during cycling. Here we analyzed crystalline silicon anodes by operando neutron reflectometry in combination with electrochemical impedance spectroscopy. The lithiation/delithiation processes were investigated over four cycles revealing a successive growth of the lithiated zone. Moreover, the loss of Coulombic efficiency could be directly correlated to a layer formation and its dissolution on the silicon surface that suppressed the insertion of lithium ions into the silicon anode. The comparison of the currents obtained by the scattering length density profiles and the potentiostat revealed that after an initial parasitic side reaction had occurred current losses of less than 5 % could be achieved. While the lithiation was hindered by side reactions the delithiation process encountered no significant problems. The results obtained by electrochemical impedance spectroscopy suggested that a layer with high charge transfer resistance was formed after each delithiation step. Hence, these operando studies provide valuable insights into the correlation of surface formation processes and the loss in Coulombic efficiency.