Institute Solar Fuels
Catalysts
Introduction
To lower the electric energy needed to split water into hydrogen and oxygen, electrocatalysts are in demand which have to be integrated in the photoactive electrode surfaces of a light driven electrolyzer. Under acidic conditions highly efficient materials are noble metal based catalysts, i.e., platinum at the cathode side as hydrogen evolving catalyst (HEC) and ruthenium and/or iridium oxides at the anode side as oxygen evolving catalysts (OEC). The water oxidation half-reaction (oxygen evolution) is especially targeted due to its mechanistic complexity and high activation energy.
Our approach
In order to make the costs for this part of a light driven electrolyzer economically viable, earth-abundant, low-cost, nontoxic and durable catalysts are required. Materials of interest as OEC are porous layers of cobalt oxyhydroxides (CoOxOHy) and manganese(III) oxide (a-Mn2O3) [1], but also more complex oxides and alloys (nickel iron oxides, molybdenum cobalt allys. Thin layers can be prepared by reactive magnetron sputtering, spin coating or electrochemical deposition techniques. To replace costly platinum catalyst molybdenum sulfides such as amorphous MoSx, carbon supported MoS2 and ammonium thiomolybdate ((NH4)2Mo3S13) are under investigation.
Recent highlights
Of special interest is to elucidate the nature of catalytically active centers of these catalysts using spectroelectrochemistry as well as synchrotron radiation techniques (soft X-ray photoelectron spectroscopy). In-operando/ in-situ techniques are used to study changes of the metal oxidation state of OEC electrodes in contact with the electrolyte under potential control. As an example, it could be demonstrated that Mn4+ ions are formed at the electrode-electrolyte interface under oxygen evolution conditions on top of a-Mn2O3 or birnessite type oxides characterized by a layer structure [ [2]-[3]].
In case of HER-catalyst MoS2, it has been demonstrated that the catalyst can electrochemically be stabilized and activated using a carbon support (multi-walled carbon nanotubes of small diameters).
Since the catalysts have to be deposited on photosensitive semiconducting electrode surfaces the compatibility of catalyst layer and semiconductor surface is an important issue [3].
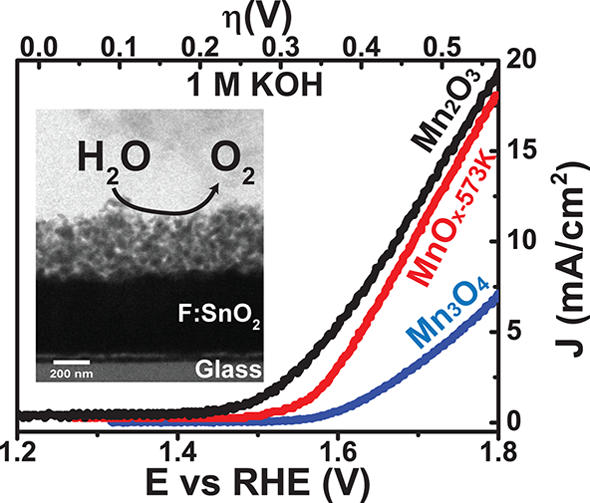
Fig. 1.
a-Mn2O3 is the most active phase in the Mn-O system as OEC. Oxygen evolution current densities of 10 mA/cm2 were achieved for highly porous, ~1 µm thick electrodeposited Mn2O3 films at an overpotential of 340 mV (pH 14). The catalytic activity is strongly affected by the large variation in Mn‑O bond lengths, Mn oxidation states, and the presence of oxygen point defects.
©HZB/Fiechter
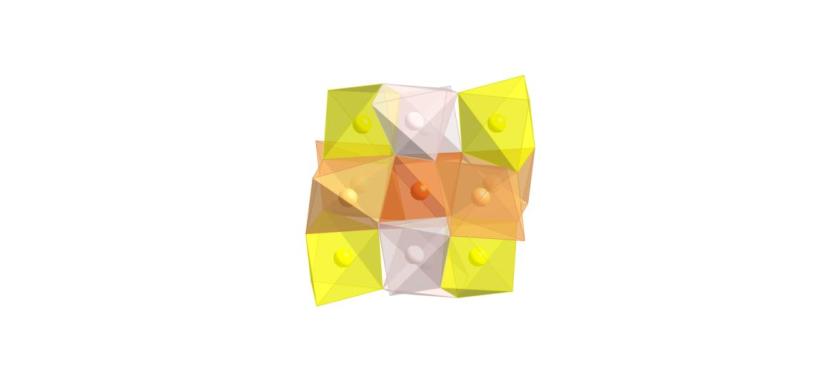
Fig. 2.
Section of the crystal structure of a-Mn2O3. Mn3+ ions are octahedrally coordinated by six oxygen atoms. The resulting d4 electron configuration of Mn3+ ion in the octahedral ligand field causes strong Jahn Teller distortion of the Mn3+O6 units leading to short O-O distances of 2.55 Å which could facilitate the O2 generation from these sites in the process of electrochemical water splitting.
©HZB/Fiechter
References
[1] M. Kölbach, S. Fiechter, R. van de Krol, P. Bogdanoff, “Evaluation of electrodeposited α-Mn2O3 as a catalyst for the oxygen evolution reaction”, Catalysis Today 2017, 290, 2-9
[2] A. Ramírez, P. Hillebrand, D. Stellmach, M.M. May, P. Bogdanoff, S. Fiechter, “Evaluation of MnOx, Mn2O3, and Mn3O4 Electrodeposited Films for the Oxygen Evolution Reaction (OER) of Water”, J. Phys Chem. C 2014, 118, 14073-14081 and SI
[3] L. Xi, Lifei, F. Wang, C. Schwanke, F. Abdi, Fatwa, R. Golnak, S. Fiechter, K. Ellmer, Klaus, R. van de Krol, K. Lange, „In Situ Structural Study of MnPi Modified BiVO4 Photoanodes by Soft X-ray Absorption Spectroscopy”, J. Phys. Chem. C 2017
