Department Atomic-Scale Dynamics in Light-Energy Conversion
Research Topics
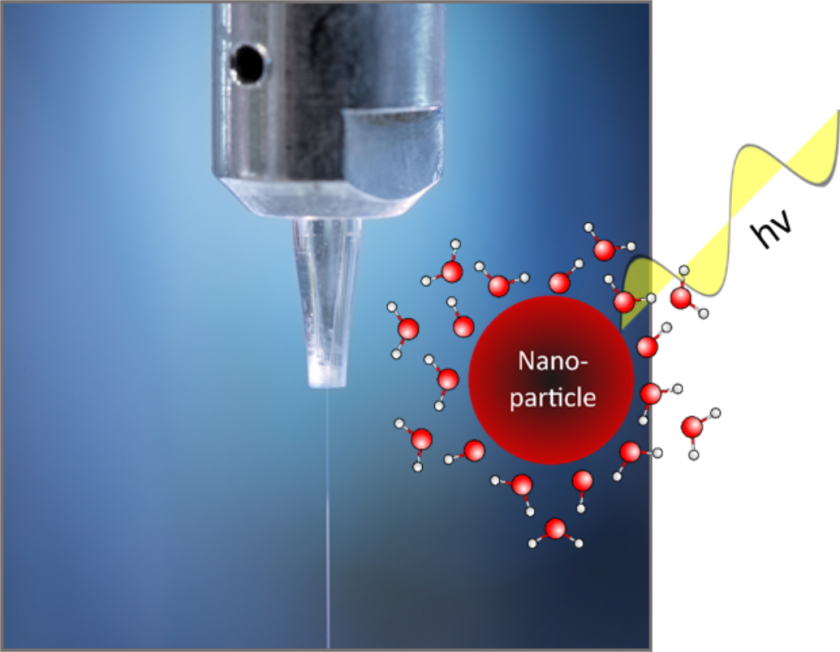
Metal-oxide nanoparticles / bulk water interaction
We investigate transition metal-oxide nanoparticles in bulk water, which are ideal model systems to mimic the electrolyte/metal-oxide anode interface in electrochemical cells under ‘open-circuit potential’ (i.e. no voltage applied). Among the most pressing questions with regard to the molecular-level understanding of such buried interfaces are how ions adsorb at the solid–solution interface, how interfacial ions are solvated, how the solvent molecules respond, and how all this affects charge-state and energy transfer across the interface. We use (resonant) liquidjet photoelectron spectroscopy. This method is sufficiently sensitive for the detection of adsorbed hydroxyl species, resulting from the water dissociation at the nanoparticle surface in aqueous solution.
Investigated nanoparticle systems/samples: Fe2O3, TiO2, FexNiyOzH, Fe3O4, CoOx, CeO2,
Publications from this topic:
ACS Applied Nano Materials 2020, 3, 264−273 DOI: 0.1021/acsanm.9b01939
Journal of Materials Chemistry A 2019, 7, 6665-6675 DOI:10.1039/C8TA09414D
Chemical Science 2018, 9 (19), 4511-4523 DOI: 10.1039/c7sc05156e
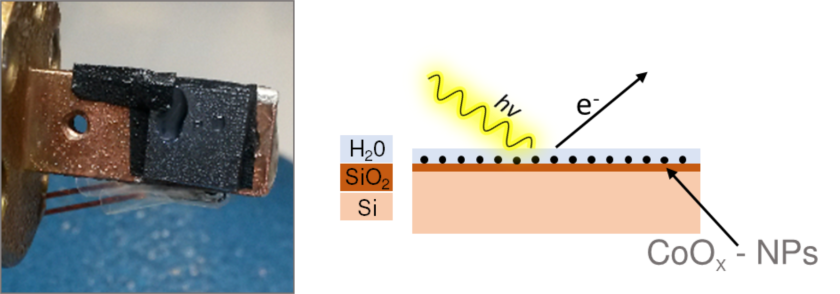
Supported metal-oxide (nanoparticles) / few-monolayer water interaction
We investigate thin-films and supported nanoparticles of (transition) metal oxides with few monolayers of water on a SiOx/Si(111) substrate. Here, we are mainly interested in the phase composition and possible phase changes at the metal-oxide surface in contact with water. We use the near-ambient pressure method to generate the few monolayers of water on top of our samples. This is accomplished by cooling the sample (holder) to 0°C inside our vacuum chamber while applying a ~4-mbar water vapor atmosphere. The thickness of the condensed water layer depends on the relative humidity in the ‘vacuum’ chamber. We use resonant photoelectron spectroscopy and partial electron yield spectroscopy (i.e. integrated resonant Auger decay signals as a function of photon energy) to reveal the subtle electronic structure differences that occur between the ‘dry’ (=high vacuum) state and when water is applied (=elevated pressure).
Investigated nanoparticle systems: CoOx, FexNiyOz(H), Fe2O3, NiOx
Publications from this topic:
ACS Nano 2020, 14, 15450-15457 DOI: 10.1021/acsnano.0c06066
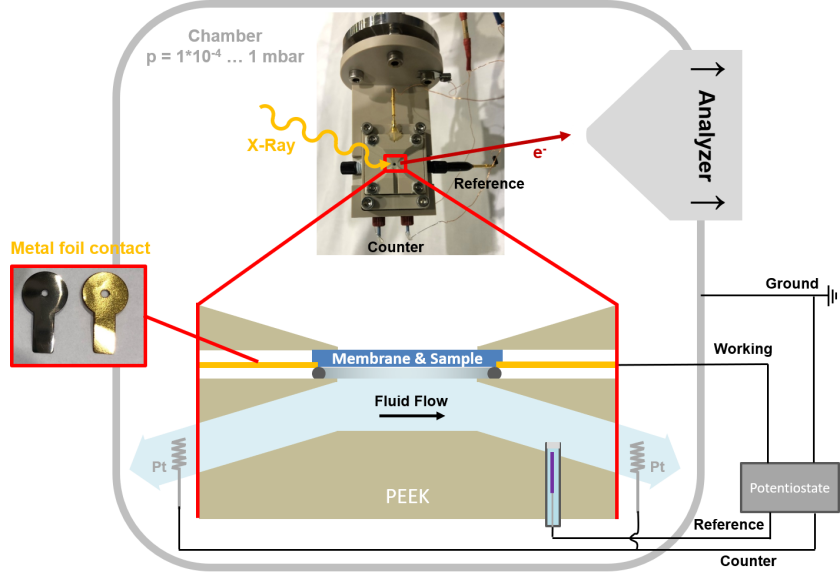
Operando resonant XPS investigation of the metal-oxide/electrolyte interface
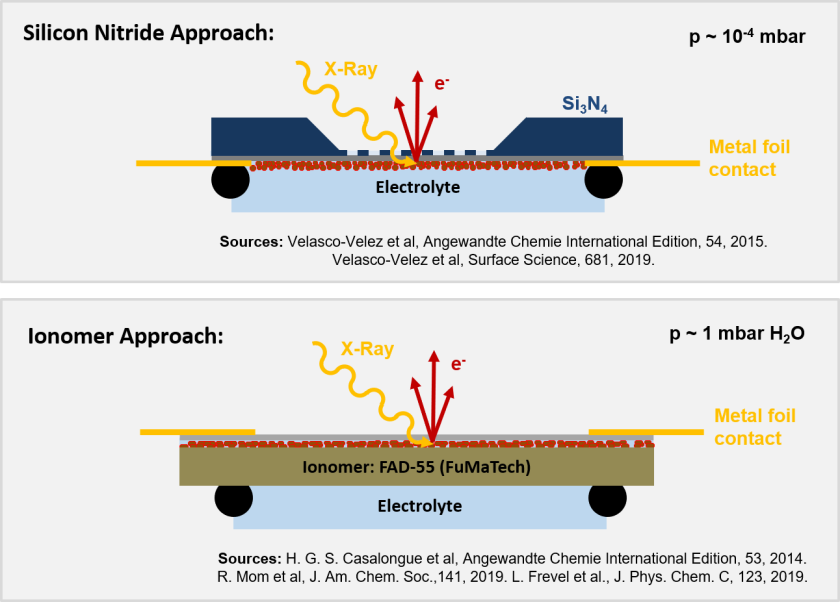
We have a huge interest in the spectroscopic investigation of nanostructured, electrodeposited iron-nickel (oxo-hydr-)oxides (FexNiyOzOH) catalysts for the oxygen evolution reaction in photo-electrochemical cells for future commercial water-splitting applications. FexNiyOzOH offers tunable parameters like the iron doping level or the oxygen and hydroxide content, which appears to be a promising model system to engineer better performing catalysts. Our primary goal is to track the water and catalyst (electronic) structure at their interface under electrocatalytic reaction conditions. We have developed an electrochemical flow cell that is based on the graphene concept pioneered at BESSY II and the FHI in the Schlögl group (Knop-Gericke, Velasco-Velez), in which a stack of graphene monolayers is deposited on top of a silicon nitride holey membrane, acting as conductive, X-ray- and photoelectron transparent window. The graphene withstands the pressure difference of 1 bar between the electrolyte and the vacuum and also acts as the anode in this micro electrochemical flow cell. Although the thickness of the graphene and the electrocatalyst is only a few nanometers, the electron inelastic mean free path is in the same order of magnitude for energies in the soft X-ray regime. To enhance the detection efficiency and to improve our signal-to-noise we mainly measure the resonant Auger spectra, after resonant excitation of the Fe 2p (~710 eV) and Ni 2p (~855 eV) electrons into the 3d band.
We also work on an alternative approach to graphene covered holey membranes. Here, the silicon nitride holey membrane is replaced by a ~50µm thick, cation-permeable ionomer. This approach was first published by the Nilsson group in 2014, and improved by colleagues at the Fritz-Haber-institute in Berlin (Knop-Gericke). The advantage is the robustness against pressure-differences, a water leakage is very unlikely. However, the sputtered electrocatalyst, being on top of the ionomor, covered with one layer of graphene is not in direct contact with the (bulk) electrolyte solution, but with confined water between the ionomer and the graphene layer. To avoid drying out of the ionomer, these experiments have to be performed at elevated pressures in a water atmosphere under near-ambient-pressure conditions.
Investigated systems: FexNiyOz(H)
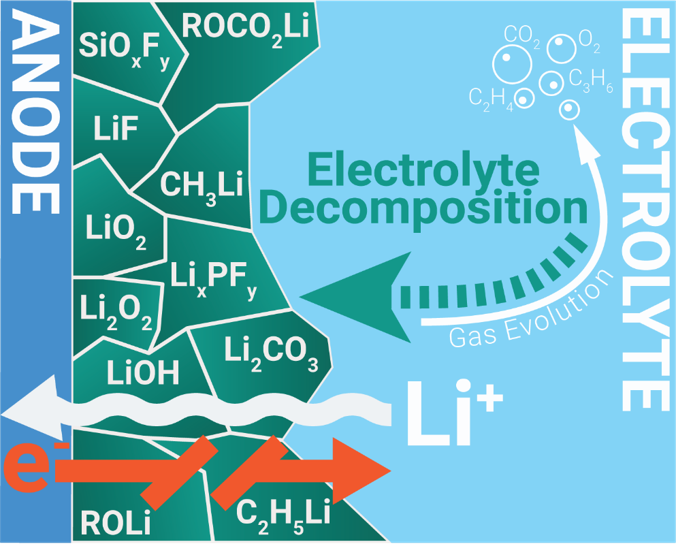
Operando transmission investigations of silicon anodes for Li-ion batteries
We investigate the formation and the composition of the solid-electrolyte interface (SEI) of silicon-anode based lithium-ion batteries. With 4200 mAh/g, silicon has an 11-times higher theoretical storage capacity than the current anode-standard, graphite, while being comparably cheap and earth abundant. However, the capacity fade poses one of the biggest challenges towards the successful application of silicon anodes in Li-ion batteries. As for the graphite counterpart, the SEI is crucial for the electrochemical performance of silicon anodes. The SEI forms during the first dis-/charge cycles of the battery through lithium reacting with the electrolyte at the anode interface. The volatile nature of the SEI and possible electrochemical relaxation processes in the conversion zone and within the SEI make in-situ and operando experiments necessary. We have developed an operando liquid cell that works in true transmission mode, which allows us to probe the conversion reaction throughout the thin film, to witness the formation of the SEI and to monitor changes in the electrolyte simultaneously. Changes in the oxidation state of silicon reveal how and when the charge compensation during the conversion reaction takes place. We also measure the oxygen K-edges after each dis-/charging cycle and try to assign the electronic features by linear combination fitting of relevant species forming the SEI.
Investigated systems: Si-based anodes in Li-ion batteries with LiPF6 in EC:DMC as electrolyte
Publications from this topic:
Material Today Advances 2022, 14, 100215 DOI: 10.1016/j.mtadv.2022.100215
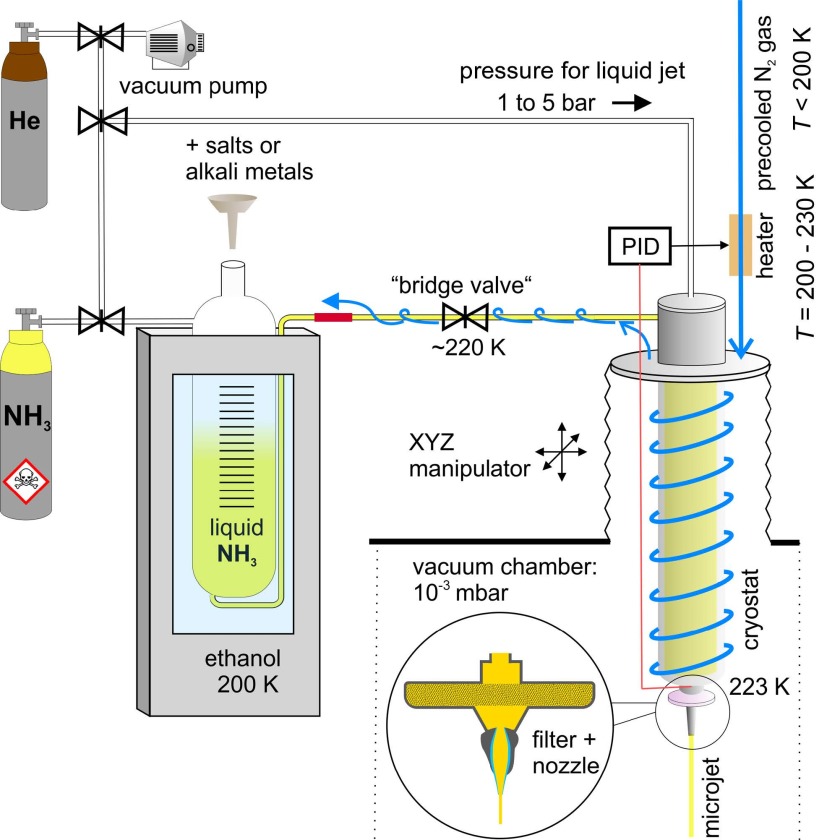
Cryo-liquid photoelectron spectroscopy
We are part of a large joint-project about the photoelectron spectroscopic investigation of solvated electrons in liquid ammonia. Our collaborators in Prague (Jungwirth group), Los Angeles (Bradforth group), and at the Fritz-Haber-institute (Winter group) have developed and improved an experimental kit to liquify gaseous ammonia and to dissolve pure alkali metals in it. The solution is then further transferred into a pressurized vessel and pushed through a micrometer-sized nozzle to form a cry liquid jet. In contrast to solvated electrons in water, solvated electrons in liquid ammonia can be concentrated up to several molar and the solutions are stable for weeks – ideal for static measurements with our SOL³PES setup. We are especially interested in the (gradual) transition from a deep blue (=less concentrated) solution, indicating an insulating solvated ‘single’ and ‘di’-electron phase, to the conducting golden metallic phase at high concentration.
Investigated systems/samples: solvated electrons in Li/Na/K – liquid ammonia solutions
Publications from this topic:
The Journal of Physical Chemistry B 2022, 126, 229-238 DOI: 10.1021/acs.jpcb.1c08172
Nature 2021, 595, 673-676 DOI: 10.1038/s41586-021-03646-5
Journal of the American Chemical Society 2019, 141 (5), 1838-1841 DOI: 10.1021/jacs.8b10942
Review of Scientific Instruments 2020, 91, 043101 DOI: 10.1063/1.5141359
Science 2020, 368 (6495), 1086-1091 DOI: 10.1126/science.aaz7607