Joint Research Group Macromolecular Crystallography
Structure of the month - February 2010
Molecular basis of antimony treatment in Leishmaniasis
Paola Baiocco#, Gianni Colotti#* Stefano Franceschini and Andrea Ilari*
Istituto di Biologia e Patologia Molecolari- CNR and Department of Biochemical Sciences, Sapienza University of Roma, , P.le A. Moro 5, 00185 Roma, Italy
# The two authors contributed equally to the work
*Corresponding Authors: Andrea Ilari, andrea.ilari@uniroma1.it and Gianni Colotti gianni.colotti@uniroma1.it
Abstract
Leishmaniasis is a poverty-related disease, deeply linked to malnutrition, humanitarian emergencies and environmental changes that affect vector biology, which is characterized by high morbidity. It causes an estimated 70,000 deaths annually, a rate surpassed among parasitic diseases only by malaria. The current treatment of leishmaniasis relies mainly on antimony-based drugs, meglumine antimoniate (Glucantime) and sodium stibogluconate (Pentostam). However, the molecular mechanism of action of these drugs was not completely understood and their use is severely impaired by the side effects related to their toxicity. In addition, an ever increasing number of drug-resistant strains have been identified which prevents the use of antimonial drugs in some endemic areas. Therefore, on the one hand there is an urgent need to develop novel drugs that target specific metabolic pathways of the parasite; on the other hand it is also necessary to understand the mechanism of action of the most used drugs in order to minimize their severe side effects.
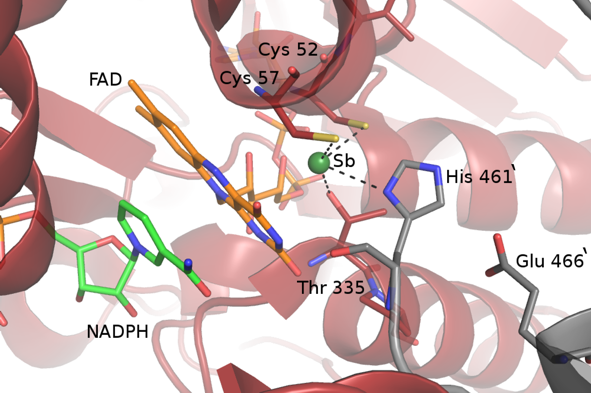
Among the potential molecular targets to date, trypanothione reductase (TR), the putative enzyme targeted by antimonial compounds, was the most promising in that it is involved in the unique thiol-based metabolism of Leishmania, which is common to all parasites of the Trypanosomatidae family but absent in the host. We solved the crystal structures of TR from Leishmania infantum in the oxidized state and of the complex of TR with NADPH and Sb(III) in the reduced state (Fig. 1A).
The structural analysis of the reduced TR in complex with NADPH and Sb(III) clearly shows for the first time that the trivalent ion binds in the catalytic cleft at the dimeric interface, engaging in complex formation the most important residues involved in trypanothione reduction, namely Cys52, Cys57 and His461’ of the two-fold symmetry related subunit, thereby inhibiting the TR activity. Sb(III) is also coordinated to Thr335 (Fig. 1B). Binding of the semimetal is only possible upon enzyme reduction because in the oxidized enzyme the two cysteine residues form a disulfur bridge.
TR inhibition abolishes the main form of regulation of Leishmania cellular redox equilibrium and impairs the parasite’s defense against oxidative stress by shifting the equilibrium towards the disulfide form of trypanothione, thereby perturbing the thiol redox potential of the cell. Since TR is essential for the parasite survival and virulence and it is absent in mammalian cells, our structural studies provide insights towards the design of new more affordable and less toxic drugs against Leishmaniasis.

Figure 1a. Overall fold of L. infantum TR. One monomer of the dimer is coloured gray. In the other monomer, the FAD binding domain (residues 1-160 and 291-360) is coloured red, the NADPH binding domain (residues 161-290) blue, the interface domain (361-488) in yellow-orange. The FAD and NADPH cofactors are indicated in stick and Sb(III) is indicated as a green sphere.